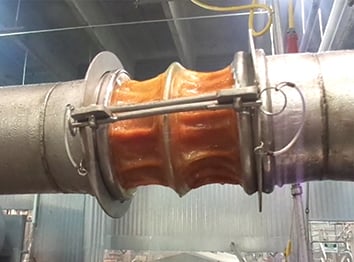
No crevices to collect product so there's no build-up or contamination risk. Cleaner plant with no leaks and safe for use in CIP.
Production environments in the food industry need to be kept sterile, and the need for cleanliness when switching product runs is crucial.
This can be particularly challenging for manufacturers running traditional clamped connector systems.
That's just one of the reasons 95% of the world's top 20 food manufacturers use BFM® fittings.
%20(2)%20(1).webp)
.webp?width=303&height=303&name=13.01_Linkedin_top20foodmanufacturers%20(1).webp)